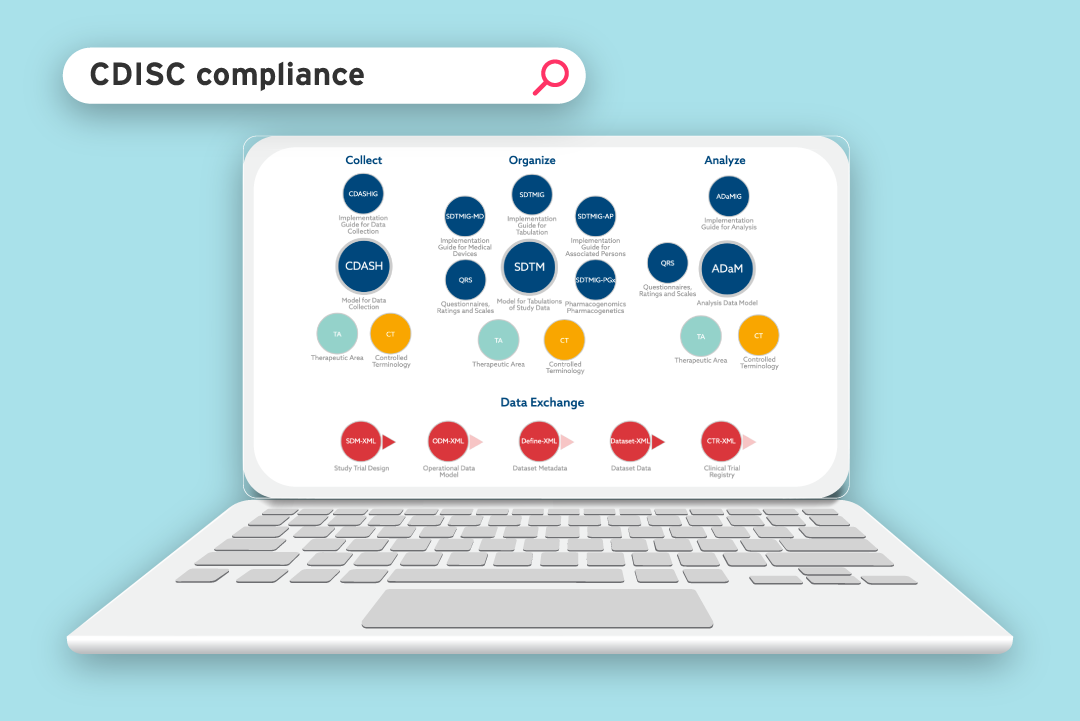
CDISC compliance is mandatory for any clinical trial submissions to the US Food and Drug Administration (FDA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA). Submissions from the academic world are no exception. In fact, many academic institutions must achieve CDISC compliance in order to be granted funding for clinical research.
CDISC standards can seem overwhelming, like a whole new language to be understood and implemented. But those institutions already implementing standardization are finding that CDISC compliance has many benefits, including improved research, data analysis and insights.
Earlier this month, we were delighted to partner up with CDISC, The University of Alabama at Birmingham (UAB) and The University of Utah to deliver a free webinar on the subject of standardization in clinical trials. Representatives from both universities discussed how CDISC standards have benefitted their organization, what they’ve learned and their future goals.
We’ve summarized the key takeaways below. You can also access the webinar recording (60 min) at the end of this blog.
Retrospective CDISC compliance
The background
Coretta (Thomas) Robinson, Program Manager, The University of Alabama at Birmingham (UAB), explained how they addressed CDISC compliance.
UAB have conducted many clinical trials, since long before compliance with CDISC standards was mandatory. Having run several trials prior to the implementation of standardization at the university, UAB was faced with the challenge of mapping metadata retrospectively to the Study Data Tabulation Model (SDTM) before submission.
As well as this immediate mapping challenge, they knew that non-compliance with SDTM could jeopardize funding for any future clinical trials. They decided to implement a plan to ensure future CDISC compliance.
UAB’s aims
- To implement CDISC standards and build datasets in line with CDISC SDTM, to enable submission to the FDA.
- To create standardized case report forms (CRFs) in line with CDASH, so future data collected would be compliant and they wouldn’t have to recreate the wheel with new forms for each study.
- To implement organizational standards and gain efficiencies through standardization.
The challenges
Lack of CDISC knowledge internally
Learning and adopting the new methods was a culture shift
Budget to invest in standardization, including for training
The requirement to retrospectively map to SDTM
As Coretta explained, academic institutions are generally not familiar with CDISC. Specifically, how the standards relate to data capture and content, and what that means for submission. This can make things like getting budget approved and getting colleagues on board with the shift, somewhat of a challenge.
However, as Coretta put it, training and working with CDISC standards is a must.
The outcome
Having learned of our CDISC SDTM expertise, UAB approached Formedix to help and we took the following steps:
- Reviewed the collected data with CDISC models in mind.
- Checked the terminology on the form matched the SDTM controlled terminology and that it could be mapped.
- Identified any potential validation issues, such as missing/blank fields.
- Reviewed the CRFs, gave feedback on the design, and aligned them to SDTM.
- Built a set of standardized CRFs that UAB could then use in future study builds.
Their investment in learning and implementing CDISC standards, with help from our experts, meant that UAB could more successfully navigate data collection, SDTM mapping and submission.
Adopting the CDISC format
Next, Russ Telford, Director of Biostatistics and Clinical Data Management at The University of Utah, explained how they adopted the CDISC format.
The background
Russ explained that it can be tempting for institutions to think retrospectively mapping to SDTM is easy, when in fact it’s anything but.
Utah did have organizational standards in place, based on Data Coordinating Centre (DCC) and National Institutes of Health (NIH) data standards. But there were conflicts between the different sets of standards, which created a headache. They also found that between trials, they often had to make modifications to the standards. It became a challenge to accurately track what changes were made to the standards and forms as they went.
They needed a way to easily and comprehensively manage their organizational standards.
Utah’s aims
Utah’s ultimate goal was to implement an end-to-end set of CDISC compliant standards, that allowed a seamless process from data acquisition to submission.
The challenges
Conflicts between standardsNo governance between standards and studies
No alignment with CDISC and National Cancer Institute (NCI) controlled terminology
Minimal reuse of standards
Divergence from standards over time
Instilling the idea of CDISC’s many benefits
The outcome
Utah approached Formedix to help, and we took the following steps:
- Performed an audit of the metadata to determine the format (OpenClinica 3).
- Used the Formedix ryze importer to pull the metadata into our system quickly and efficiently, saving a lot of time. And as that importer is pre-validated for OpenClinica 3, quality checking was straightforward.
- Organized the raw standards by topic (demographics, adverse events, pain). The integrity of the source data remained intact, for traceability purposes.
- Performed a cross-study comparison to determine what forms to keep and what to discard.
- Created a new eCRF library, primarily based on CDASH variables and NIH controlled terminology, with some content from old DCC and NIH standards where required. This allowed ryze to identify what had changed.
- Reviewed and approved standards using ryze visualizations, so they can now be used and reused consistently across studies.
Russ explained that in implementing CDISC, they now have a universal standard they can rely on, and that allows increased efficiencies across their studies. They are seeing efficiencies in study start up, the reuse of standards and controlled terminology. They are also seeing benefits beyond this, in respect of post study datasets, including efficiencies in statistical analysis and reporting, and in their submissions.
The benefits of CDISC compliance
Both Coretta and Russ agree that none of the challenges of implementing CDISC compliance are insurmountable, and that the benefits of adopting the CDISC format are well worth the effort.
CDISC standards provide a common structure for data collection, analysis and submission, and adoption of the standards has many benefits:
- Greater efficiency
- Better interoperability
- Higher data reuse
- Better data quality and traceability
|
Access the CDISC knowledge base, a useful portal containing articles, FAQs, known issues and form examples. |
For more information about the standards themselves, read our helpful guide to CDISC standards used in the clinical research process.
 ryze and CDISC
ryze and CDISC
Win more trials, bid for more grants, save time and resources, and increase your chances of FDA approval with ryze clinical metadata repository and automation suite.
ryze is built on CDISC data standards, so compliance with CDISC SDTM, ADaM, and controlled terminology is taken care of. ryze even prompts you if you stray from the standards.
|
Some of our clients see as much as 94% reuse of study metadata while using ryze. |
Learn more about how ryze can help you comply with CDISC data standards.
Listen to the webinar
To hear more from Coretta and Russ, and from representatives from CDISC on the standards themselves, listen to the webinar in full by clicking the button below.
![]()
About the author

Gilbert Hunter
Customer Success Manager | Formedix
Gilbert joined Formedix nearly ten years ago as a technical writer. The system knowledge he gained from content development, together with his existing customer service skills, marked him out for transition to the Professional Services (PS) team.
Gilbert has worked with the PS team for over four years, providing both CDISC-based training and software training, as well as support and consultancy services to Pharmaceutical, Biotechnology and Clinical Research Organizations. He helps organizations build studies faster and to a higher quality by making their clinical trial design and regulatory submissions far more efficient.
Today, as Customer Success Manager, Gilbert’s focus is to ensure customers maximize the benefits they can achieve from ryze by overcoming their challenges and achieving their goals.