What are case report forms?
A case report form (CRF) is a document designed to record all patient information that’s required during a clinical trial. The terms ‘CRF’ and electronic CRF or ‘eCRF’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’.
CRFs play a crucial role in helping to assess the safety and efficacy of clinical products. For a study to be successful, the data collected must be correct and complete. This means that forms must be well planned with meticulous attention to detail. They must comply with the study protocol and record all the required details. They must also comply with regulatory requirements, such as those defined by the US Food and Drug Administration (FDA).
The key to a good CRF is in the design process. With improvements in technology over the last 20 years, there’s been an industry-wide shift towards designing and maintaining eCRFs using cloud-based systems like CMDRs and EDCs (electronic data capture systems). By creating CRFs in a centralized platform you can ensure your teams are working from the latest, approved versions of forms. Editing and updating can be done far more easily and forms can be reused, meaning future trials are built quickly and more efficiently.
Designing CRFs in clinical trials
Good CRF design is essential for a successful submission, and for getting clinical products to market quickly.
We’ve found that organizations often struggle with the following design challenges:
- Creating forms that are consistent
- Creating user-friendly forms
- Collecting precise data
How do I design quality CRFs?
Here are some best practice steps to overcome these common challenges:
- Do proper planning early in the study. This should be done by a team of people that includes data managers, biostatisticians, and clinicians.
- Define clear objectives and stick to them – what information are you required to gather?
- Maintain standardized forms – that way, you can reuse the form over and over.
- Get user feedback. It’s best to build this into the design and maintenance process.
- Provide form completion guidelines with specific instructions to reduce data entry issues and ambiguities. This helps investigators fill in forms correctly and ensure that data is accurately captured for analysis.
Why should CRFs be standardized?
First, what do we mean by standardized CRFs?
Standardization is the process of developing content to meet specific regulatory and organizational standards. Standardized forms will conform with regulatory requirements i.e. be laid out and annotated as required by CDISC’s Clinical Data Acquisition Standards Harmonization (CDASH) standard. They will also conform with the requirements of different internal stakeholders within the study team, as stakeholders are given the chance to review and approve the forms for use.
There are many benefits to standardizing your CRFs:
- Standardization means creating forms that cover everything your stakeholders need; investigator, data manager, biostatisticians and data entry personnel can all review and approve the standardized form.
- This means standardized forms can be used again and again, saving time on both design and approval in future studies.
- Standardized forms are compliant with regulatory requirements, so elements such as annotations don’t need to be manually done on each form in the study, saving time and effort.
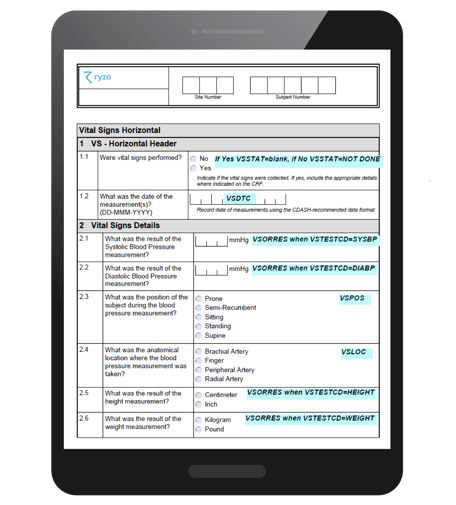
What are annotated CRFs?
Annotated CRFs are a key submission deliverable, a mandatory requirement of the FDA. Each form in a study contains markings or annotations. These annotations map data points on forms to the name of datasets, and variables within those datasets. In other words, “each CRF should provide the variable names and coding for each CRF item included in the data tabulation datasets” as stated by the FDA guidelines.
|
The FDA stipulates that a set of blank annotated CRFs be submitted in a PDF document called “blankcrf.pdf”. This document helps the FDA reviewer find the origin of variables in the SDTM datasets. |
You can read more about why it’s a good idea to switch to automated CRF annotations.
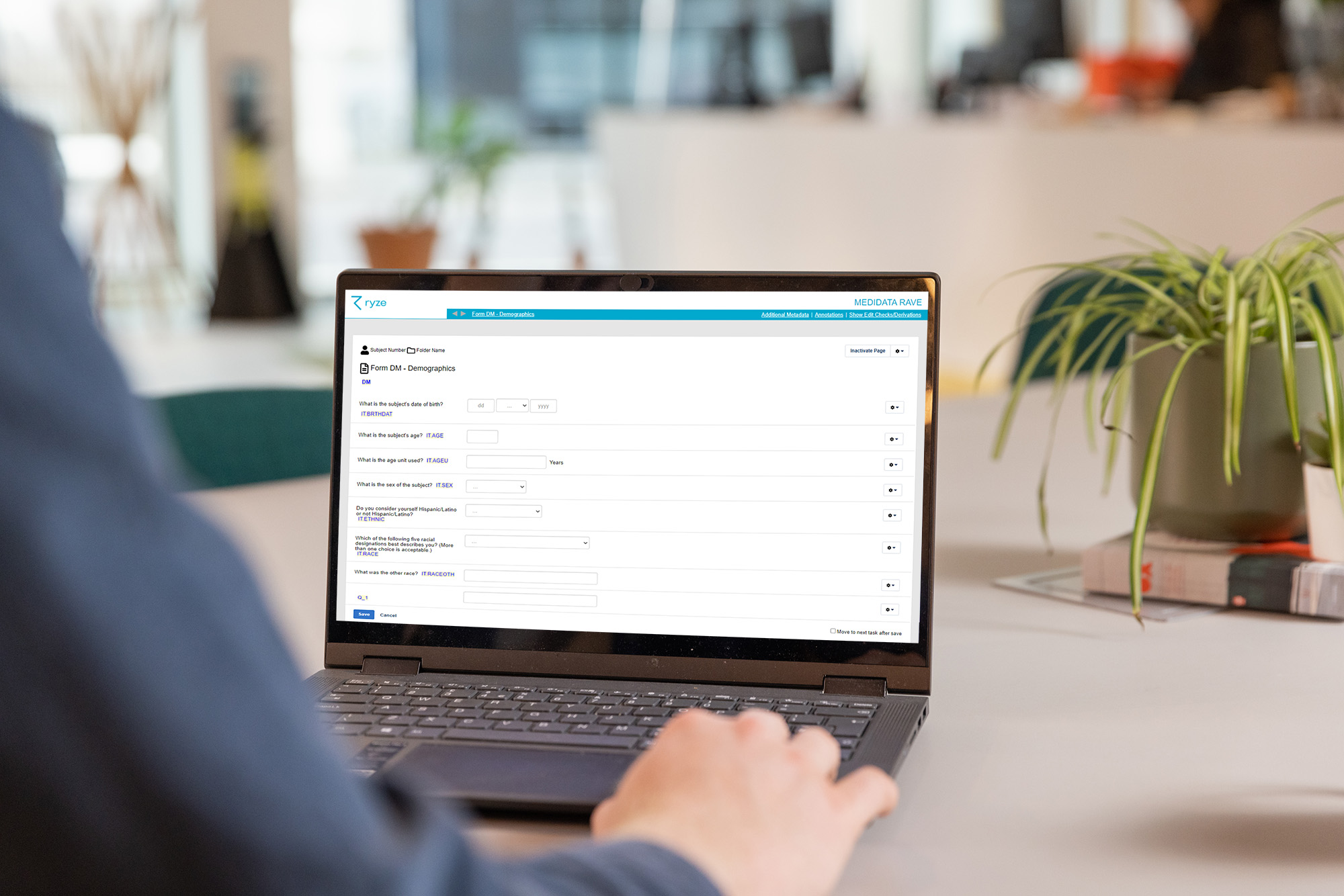
Here’s an example of an annotated CRF in ryze.
Creating good quality annotated case report forms (CRFs) takes time and attention to detail. While it can feel overwhelming, there is industry and regulatory guidance to help you get it right. Read our blog about the guidance that’s available to help ensure you’re meeting the requirements.
If you want to learn more about CRF design, why not download our guide to common case report form design issues and how to solve them? Our guide also includes a CRF design checklist to keep you on track!
Author's note: this blog post was originally published in January 2021 and has been updated for accuracy and comprehensiveness.
![]()
About the author

Gilbert Hunter
Customer Success Manager | Formedix
Gilbert joined Formedix nearly ten years ago as a technical writer. The system knowledge he gained from content development, together with his existing customer service skills, marked him out for transition to the Professional Services (PS) team.
Gilbert has worked with the PS team for over four years, providing both CDISC-based training and software training, as well as support and consultancy services to Pharmaceutical, Biotechnology and Clinical Research Organizations. He helps organizations build studies faster and to a higher quality by making their clinical trial design and regulatory submissions far more efficient.
Today, as Customer Success Manager, Gilbert’s focus is to ensure customers maximize the benefits they can achieve from ryze by overcoming their challenges and achieving their goals.