Streamlining metadata management is crucial for successful clinical trials. Inconsistent, outdated, or unreliable information can make it difficult to find the right forms and ensure compliance with current internal standards, as well as regulatory standards.
What Exactly is a Clinical Metadata Repository?
A clinical metadata repository is a centralized library that stores and manages data definitions like forms, codes, and datasets, improving data quality and streamlining clinical trials.
By adopting a comprehensive clinical metadata repository, organizations can enhance the management of their metadata, which leads to higher quality submission data, quicker access to data insights, and faster introduction of new drugs to the market.
What Do We Mean by Metadata in Clinical Trials?
In clinical trials, metadata consists of essential components like forms, terminologies, datasets, and mappings. It's the information that describes data, such as the measurement variables (e.g., height, weight) and their characteristics (numeric or text) helping ensure accurate and consistent data collection.
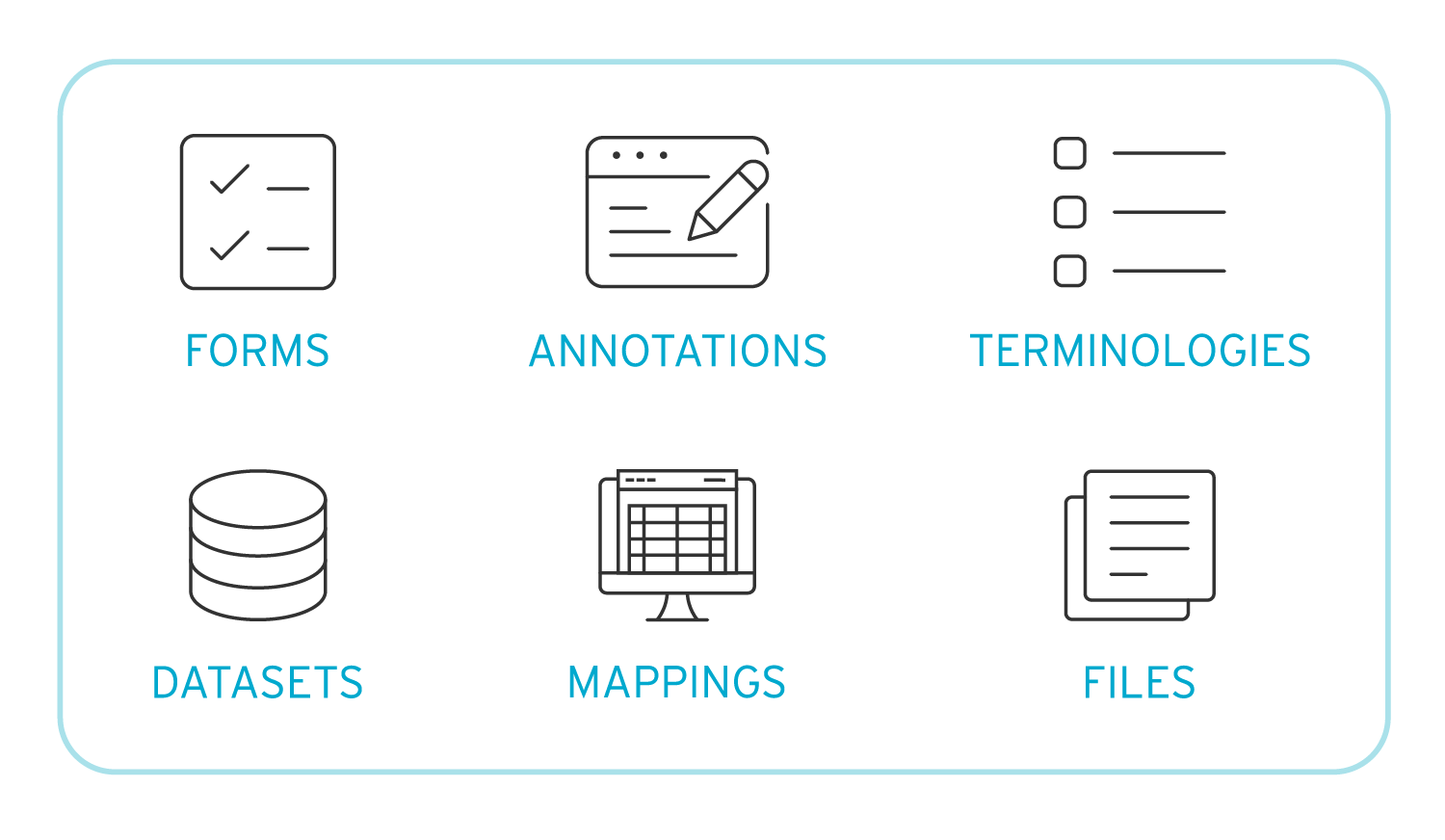
What are the Benefits of Implementing a Clinical Metadata Repository?
Implementing a clinical metadata repository enhances data quality by standardizing data collection methods, allowing early and informed decision-making, and promoting collaboration. By centralizing and standardizing metadata, you can access and utilize data more efficiently, improving patient outcomes and accelerating drug discovery.
What Should You Consider when Choosing a Clinical Metadata Repository?
Selecting the optimal clinical metadata repository goes beyond basic functionality. While features like Clinical Data Interchange Standards Consortium (CDISC) compliance, version control, and easy metadata retrieval are essential, not all platforms offer the same level of capability.
To maximize your investment, make sure that your clinical metadata repository aligns with your specific needs and provides advanced features for efficient clinical metadata management.
What Basic Functionality Should a Clinical Metadata Repository Have?
The following functionalities are a ‘no-brainer.’ A clinical metadata repository should, as a minimum:
- Give you easy access to CDISC-compliant templates to make study build faster and easier CDISC-compliant.
- Stay up to date with new versions of the standards and support older versions.
- Let you reuse your existing standards to save time and ensure consistency in your data collection.
- Have version control to track changes and revert to previous versions if necessary.
- Have built-in traceability to follow the origin and transformations of your data, ensuring its accuracy.
- Let you find your metadata easily and quickly with powerful search features to locate specific metadata elements.
Additional Features of a Clinical Metadata Repository
For optimal clinical trial management, additional clinical metadata repository features are important:
Visibility
It’s important to know exactly how your metadata is being used throughout your clinical trial. A metadata repository should provide clear visibility of:
- Where and how your metadata is used
- How often it’s used
- The impact of changing it
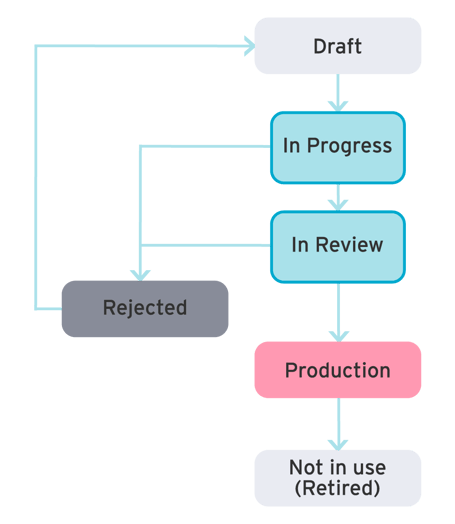
Governance
Effective governance ensures metadata accuracy through clear lifecycle management, enhancing data quality and regulatory compliance.
Collaboration
A clinical metadata repository should support seamless collaboration across different roles and locations, ensuring transparency and consistency in data management.
EDC Integration
A central metadata repository, seamlessly integrated with your EDC (electronic data capture) system, is vital for efficient clinical trial design. It allows for building studies directly from standardized templates, including complex CRFs (Case Report Forms) with edit checks. This streamlines the entire process, saving time, reducing errors, and ensuring consistent, high-quality data.
How to Implement a Clinical Metadata Repository?
Implementing a clinical metadata repository can revolutionize clinical trial management. Streamlining internal processes and enhancing study-build efficiency can facilitate high-quality data management and expedited drug development. However, choosing the right clinical metadata repository is crucial.
By focusing on essential functionalities like CDISC compliance and version control while also considering advanced features for specific needs, you can unlock the full potential of clinical metadata repositories, leading to more efficient clinical studies and quicker treatment delivery to patients.
How do you implement a clinical metadata repository?
We’ve explored the different functionalities of a clinical metadata repository, and how some added functionality can elevate your clinical trials and make them far more efficient. We’ve shown you that, by leveraging the power of a centralized clinical metadata repository, you can improve your internal processes, build faster, more efficient clinical studies, and accelerate the delivery of new treatments to market.
Hopefully by now, you’ll have a good idea of how a clinical metadata repository can improve your study build processes, and what to look for in any clinical metadata repository.
Are you ready to implement your clinical metadata repository? Then you’ll want to download our free quick guide to implementing a CMDR! In the guide, we cover:
- Choosing a CMDR that’ll help you achieve efficiencies in study build
- How to ensure stakeholders needs are met during implementation
- The approach you should take when standardizing metadata, to reduce effort and time spent on study set up










