Clinical trials in drug discovery
Clinical trials have formed part of medical research for decades. And in recent years, trials have become widely accepted as the most effective way to assess different methods of disease prevention and treatment. While animal testing and computer-simulated testing do provide useful results, human clinical trials are arguably the most reliable way to discover new safe and effective medicines.
Clinical trials can be used to test:
- new vaccines to prevent the spread of illness
- new treatment methods and new drugs
- improved surgical procedures or tools
- improved methods of physical therapy
- improved methods of diagnosis, for example through scanning or blood tests
- advanced treatment of psychological conditions
The impact of clinical trials
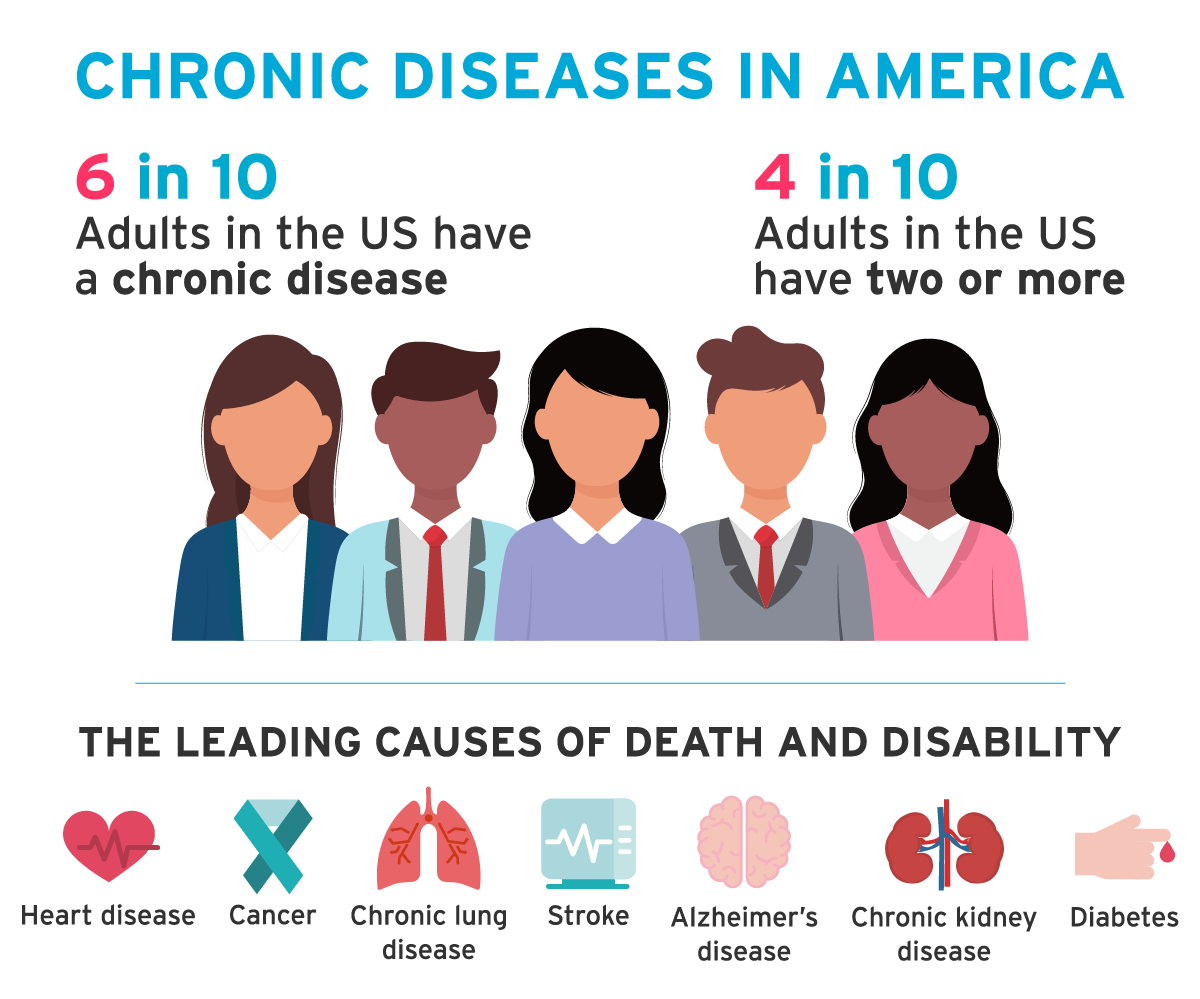
According to the Centres for Disease Control and Prevention, 6 in 10 adults in the United States have a chronic disease such as cancer, heart disease, or diabetes. The treatment that these individuals receive will have resulted from medical research to some degree.
In September 2019, Oxford University Press published a study into how the discovery of new drugs has improved life expectancy across 27 countries. The study concluded that without the development and release of new drugs from 1981 to 2013, the number of years of life lost in individuals under 85 would have been 2.16 times higher.
In May 2021, Drug Discovery and Development published a list of 25 promising pipeline drugs. It asserts that each one ‘hold[s] promise for treating conditions ranging from cancer to COVID-19 to diabetes’.
This list includes new drugs in the treatment of HIV infection, Parkinson’s disease, Alzheimer’s disease, and Leukaemia. While some of these treatments are experimental, and others are in the early phases of testing, each one is made possible by the process of medical research, including clinical trials.
The process of discovering new drugs is constantly evolving in order to provide better treatment options and ways to operate. It’s clear that access to new, more effective treatments has transformed the healthcare industry. Many lives have been saved and patients’ quality of life has improved over the years. And yet, clinical study build continues to be a very time-consuming and complex process.
Clinical data standards
In August 2022, ClinicalTrials.gov lists over 423,000 registered clinical studies across all 50 U.S. states and involving 220 countries worldwide. That’s an impressive number of life-saving or life-enhancing treatments that may become available in years to come! However, it can be as long as 18 years from the start of the trial process before new treatments reach patients.
Every clinical trial must adhere to rigorous standards in order to be successful. The US Food and Drug Administration (FDA) requires clinical study data to be submitted in a standardized format, enabling them to process, review, and archive submissions efficiently. In collaboration with the FDA, the Clinical Data Interchange Standards Consortium (CDISC), developed data standards to be applied at each step of the study process, from design to data collection and analysis.
The CDISC SDTM framework is used for organizing data collected in all human and animal clinical trials. Find out all you need to know about SDTM, including information on SDTM mapping and SDTM datasets.
Adherence to this framework will help clinical studies become a success. But it requires companies to have a thorough understanding of the standards themselves and to ensure compliance throughout study design and build. This is where software for clinical trials can help to speed up the process.
COVID-19 and accelerated drug discovery using clinical study software

When the COVID-19 pandemic hit, the pharmaceutical industry had to accelerate the usual process of drug discovery. Within weeks of vaccine development, multiple studies were underway to determine viability of new treatments.
Phase I and II data was available from as early as July and August 2020. This was partly due to the swift conversion of raw data to SDTM files.
Standardization was crucial to ensuring that data could be delivered to sponsors on a daily basis. It meant that insights could quickly be drawn and the trial could be adapted accordingly. Therefore, the standardization of data considerably reduced the time needed to bring vaccines to market.
Automation software for clinical trials and clinical metadata repositories (CMDRs) were instrumental in the success of several high-profile COVID-19 clinical studies. They enabled manual tasks to be automated and metadata to be reused across studies. Not only that, they also allowed raw data to be rapidly converted to SDTM, ready for submission.
Software for clinical trials
Specialised software for clinical trials such as CMDRs can help make clinical trials more efficient and get safe and innovative treatments to those in need quickly.
ryze, the only all-in-one cloud-based clinical MDR, can help organizations:
- Manage studies and standards from one platform, to be reused as often as you like; save time previously spent finding, consolidating and standardizing data from disparate files and systems.
- Design CRFs within ryze and instantly see how they look and work. Then make and view changes directly in the ryze platform. When you’re happy, it’s just one click to build your EDC.
- Rapidly design and build studies for leading EDCs – when you build your study in ryze, the build file contains all the EDC specific functionality, including edit checks and visit schedules. And, ryze automates the build process, saving time, so you can start your trials much sooner.
- Build studies in a fraction of the time it normally takes – it can be as little as 6 weeks!
- Stay compliant with CDISC clinical data standards including SDTM, ADaM, SEND, and NCI Controlled Terminology so that you easily align with FDA requirements.
- Design edit checks, EDC specific metadata, 3rd party lab transfer specs, mapping metadata, analysis results metadata and more.
- Transfer metadata between various platforms and continually reuse it. So, if you switch EDCs, there’s no need to redesign, retype, or retest content. Just move your assets and reuse them.
- Have greater control and traceability, so you can see how content has changed over time.
- Rely on built-in impact analysis, so that you can see the impact of any changes and easily identify any content that needs to be updated in response to a change.
- Get instant visibility of patient data once it’s live in the EDC to reduce time, labour and programming skills.
- Speed up SDTM conversion with SDTM data mapping automation tools – then get your SDTM datasets at the click of a button! Download our free guide on how to solve SDTM implementation problems.
Learn more about ryze
ryze could help you build studies faster, and more efficiently, so that life saving and life enhancing treatments get to those that need it most more quickly. Ready to learn more about clinical study software? Click the button below.