Did you know that the first randomized clinical study was conducted back in 1747 by Scottish doctor, James Lind? Lind determined through an experiment conducted onboard a naval ship that oranges and lemons could be used to cure scurvy in sailors.
Now, on May 20th every year the world celebrates Clinical Trials Day to mark the anniversary of the very first randomized clinical study. It’s a day to recognize the efforts and achievements of clinical research professionals across the globe, and to thank them for their work in improving public health.
Let’s take a look at what the clinical research industry looks like 276 years on from Lind’s experiment…
What are clinical trials?
The US National Cancer Institute (NCI) defines a clinical trial as ‘a type of research study that tests how well new medical approaches work in people’.
Clinical trials are widely accepted as the most effective way to assess different methods of disease prevention and treatment.
Clinical trials are used to test:
- new vaccines
- new drugs
- new or improved treatment methods (eg surgical procedures and tools)
- improved physical therapy techniques
- advanced treatment of mental health conditions
- improved methods of diagnosis
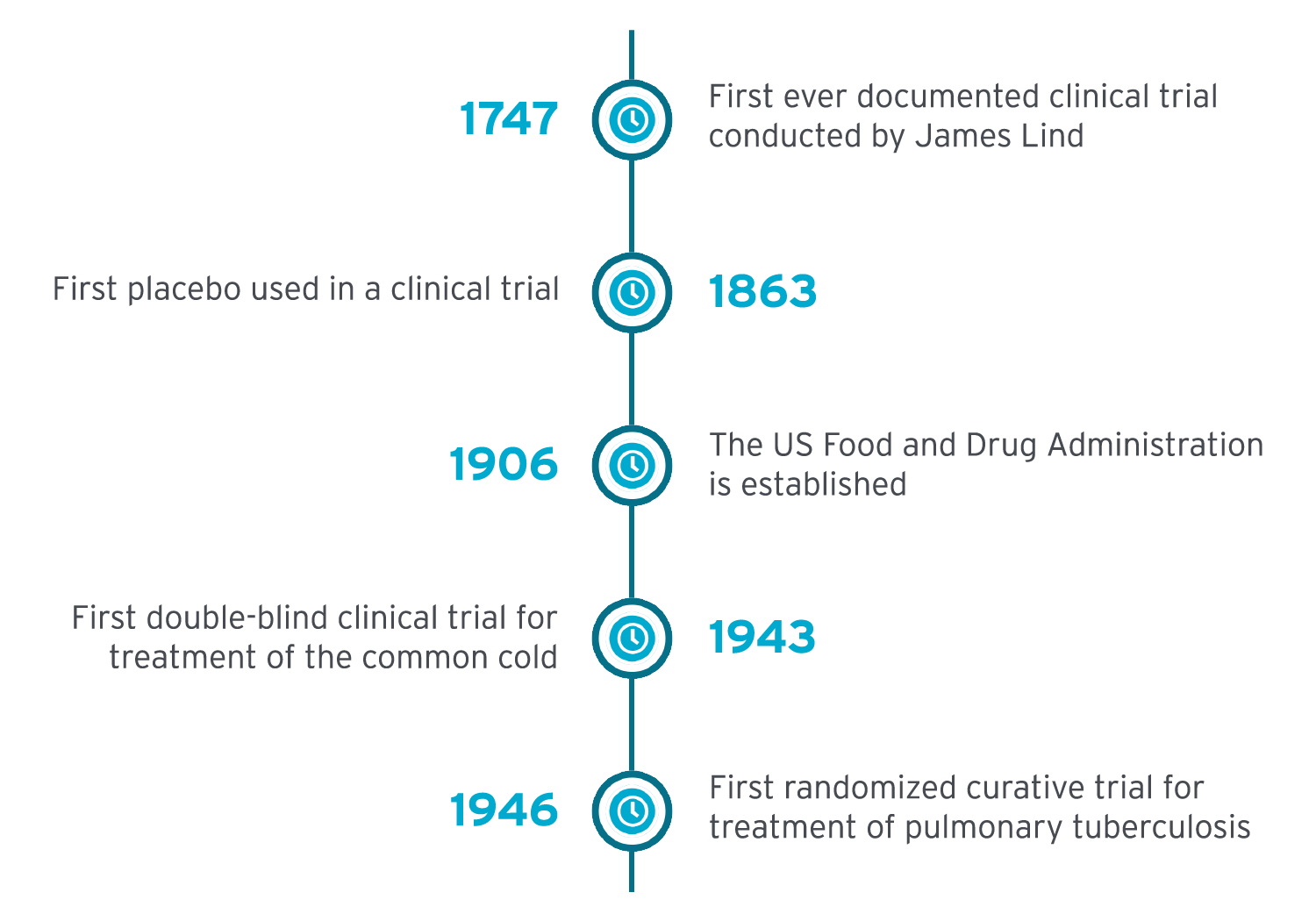
Clinical trials timeline
Clinical trials have formed part of medical research for decades. Here’s a brief history of key clinical trial milestones:
1747 - First ever documented clinical trial conducted by James Lind
1863 - First placebo used in a clinical trial
1906 - The US Food and Drug Administration (FDA) is established
1943 - First double-blind clinical trial for treatment of the common cold
1946 - First randomized curative trial for treatment of pulmonary tuberculosis
Placebo: The NCI defines a placebo as ‘an inactive substance or other intervention that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or other intervention are compared to the effects of the placebo.’
Double-blind clinical trial: According to NCI, in a double-blind study ‘neither the participants nor the researcher knows which treatment or intervention participants are receiving until the clinical trial is over’.
Randomized curative trial: The NCI defines a randomized trial as ‘a study in which the participants are divided by chance into separate groups that compare different treatments or other interventions. Using chance to divide people into groups means that the groups will be similar and that the effects of the treatments they receive can be compared more fairly. At the time of the trial, it is not known which treatment is best.’
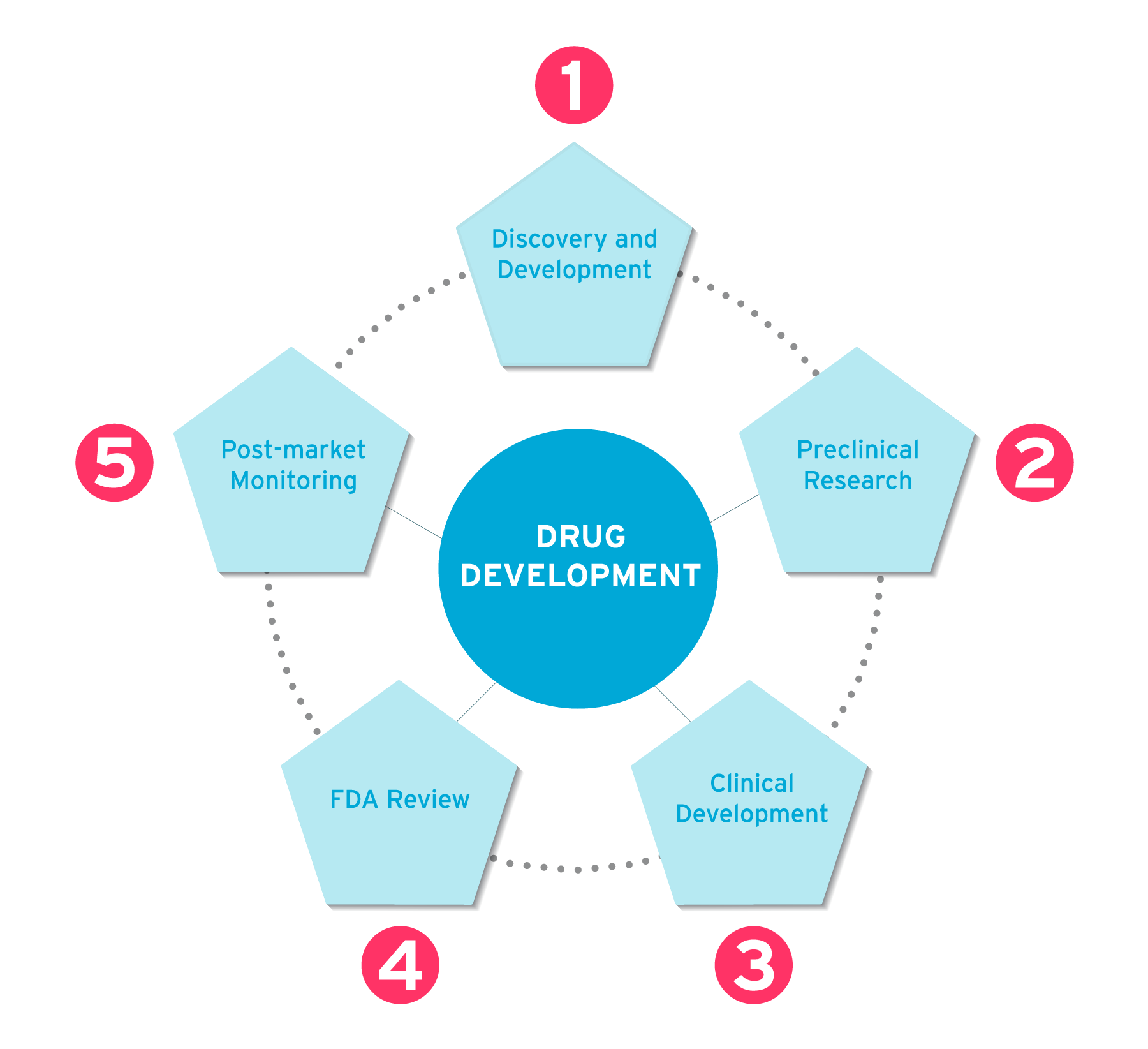
Clinical trial phases
All clinical trials follow a set process, one that’s designed to test the efficacy of the new treatment and to make sure it’s safe to use. This process is split into five phases:
Phase 1 Discovery and development - Laboratory research into the new drug or treatment method.
Phase 2 Preclinical research - Laboratory and animal testing to examine the safety of the drug.
Phase 3 Clinical development - The treatment is tested on humans for safety and efficacy.
Phase 4 FDA review - Regulatory review of all collected and submitted data, and the decision is made about whether to approve the treatment for use.
Phase 5 Post-market monitoring - The treatment is available for public use and the regulator monitors the drug to ensure it continues to be safe and effective.
You can find out more about what’s involved during the different clinical trial phases on the FDA website.
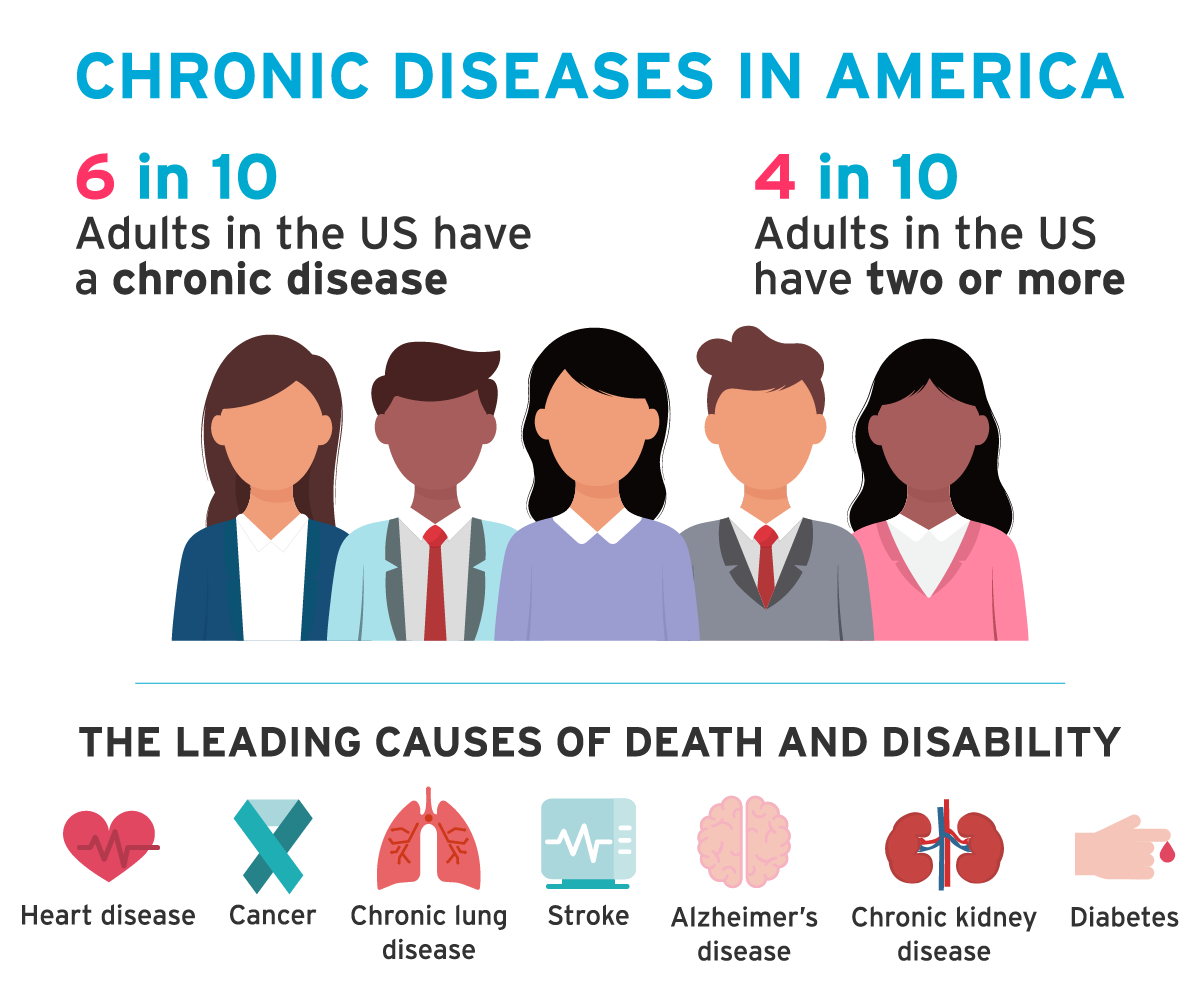
The need for clinical trials
According to the Centres for Disease Control and Prevention, 6 in 10 adults in the United States suffer from a chronic disease such as heart disease, diabetes, or cancer. The treatments offered to these individuals will have resulted to some degree from medical research. It’s clear that the work of clinical research is far reaching, and that clinical trials have transformed patient’s lives and the global healthcare industry as a whole.
But the work is never done; the industry is constantly striving to reach further and to do more for patients. Just this month, the National Institutes of Health announced a partnership with pharmas, biotechs and not-for-profit organizations to add eight rare diseases to its trial portfolio, offering hope to individuals living with these rare diseases that there may one day be treatments available. And there’s a drive in the industry to improve representation in clinical research, to help increase confidence in new treatments among marginalized communities.
Clinical research is only possible because of the medical professionals who research and run the trials, and the volunteers who take part. If you’re interested in taking part in a clinical trial, these resources can help you find out more:
- If you’re in the UK, Be Part of Research has lots of information on clinical research and how to take part.
- If you’re in the USA, visit the National Institutes of Health website for advice on how to volunteer.
- The World Health Organization provides information about accessing clinical trials in countries all around the world.
The future of clinical trials
In recent years, clinical trials have become more important than ever; only through the acceleration of clinical trials was the industry able to so quickly develop the vaccinations required to get the world back to normal after the COVID-19 pandemic. In fact, COVID-19 vaccinations were developed in just 11 months.
As we’ve seen, the clinical research industry has come on leaps and bounds since James Lind’s discovery, and there’s much to be positive about for the future of medical advancement.
According to Cancer Research, it can take 10 to 15 years for life-saving or life-enhancing drugs to be tested and approved. Like any business, it’s important for the clinical research industry to embrace innovation, technology and automation to help speed up the clinical trial process and get treatments to market faster.
| Did you know |
| Following Lind’s discovery, it took 42 years for the UK Government to issue an order that made the supply of lemon juice to sailors compulsory on ships. |
At Formedix, we’re proud to help pharmas and biotechs bring new life-saving therapies to patients much quicker. In fact, our clinical metadata repository and automation platform was used to help speed up COVID-19 clinical trials back in 2020.
To find out more about the need to accelerate drug discovery, why not listen to our founder and CEO Mark Wheeldon speaking last year on the innovative podcast ‘Transformation in trials’? Mark discusses everything from the early history of clinical data standards to the innovative technology now used to make clinical trials more efficient.









