Tags:
Annotated CRFsCreating good quality annotated case report forms (CRFs) takes time and attention to detail. While it can feel overwhelming, it shouldn’t stop you. Annotated CRFs (aCRFs) are a mandatory requirement of the Food and Drug Administration (FDA), and therefore a key submission deliverable for any clinical trial.
So, it’s important to understand what regulatory and industry guidelines are out there, to ensure your clinical trial meets the requirements.
What is an annotated CRF?
Each form in a study contains markings or annotations. These annotations map data points on forms to the name of datasets, and variables within those datasets. According to the FDA guidelines, “each CRF should provide the variable names and coding for each CRF item included in the data tabulation datasets”. Read everything you need to know about CRFs or find out what to consider when working with CRFs in clinical trials.
Below is an example of an annotated CRF that adheres to regulatory and industry requirements.
![set-of-PC-laptop-phone-screens-[Converted]](https://blog.formedix.com/hs-fs/hubfs/set-of-PC-laptop-phone-screens-%5BConverted%5D.png?width=1500&name=set-of-PC-laptop-phone-screens-%5BConverted%5D.png)
Regulatory requirements
So how do you achieve submission-ready aCRFs?
There are two documents published by the Food and Drug Administration (FDA) that include requirements applicable to annotated CRFs.
We’ve highlighted some key points from both documents below. We recommend that you download and review each PDF thoroughly, to ensure adherence to requirements.
FDA’s study data technical conformance guide section 4.1.4.6 lays out guidance for providing regulatory submissions electronically, as follows:
- aCRFs should be submitted ideally at the same time a protocol is submitted.
- The aCRF should be in PDF format, and should have the file name ‘acrf.pdf.’
- aCRF should include treatment assignment forms, if relevant, and should map each variable on the CRF to the corresponding variables in the datasets or database.
- For each CRF item, include the variable names and coding.
- When data are recorded on the CRF but are not submitted, the CRF should be annotated with the text ‘NOT SUBMITTED.’ Include an explanation in the relevant reviewer’s guide stating why these have not been submitted.
FDA’s portable document format (PDF) specifications guide; as the annotated CRF is a PDF, it is subject to the specifications in this document.
The following technical requirements are applicable to the formatting of annotations on CRFs – this list includes some key requirements, but is not exhaustive. Review the PDF download for a full list of technical requirements for submitted PDFs, including guidelines for page layout and document navigation.
- Submitted PDF files should be in PDF versions 1.4 through 1.7, PDF/A-1 and PDF/A-2 and readable in Adobe Acrobat.
- Documents should not be password protected.
- Only standard fonts should be used at 9-12pt, and any non-standard fonts should be fully embedded. You can see a full list of standard fonts in the PDF download.
- It’s recommended that text should be black, but blue is permitted for hyperlinks.
- The PDF should be text searchable, and should include a contents table featuring hyperlinks to bookmarked pages.
Industry requirements
In addition to the regulatory requirements for annotated CRFs, you should also be aware of industry requirements from the Clinical Data Interchange Standards Consortium (CDISC).
These are laid out in the following documents:
CDISC’s annotated CRF SDTM guidelines
CDISC’s metadata submissions guidelines (MSG)
Below, we’ve summarised some key points as recommended by these documents. We do advise that you review the PDF downloads, which contain detailed guidance.
- The aCRF should be bookmarked in two ways ‒ chronologically and alphabetically.
- The aCRF must not contain any blank pages.
- Each domain that is represented on a page should have its own annotation on the upper left side of the CRF page with the 2-letter domain code and domain name.
- If more than one domain exists on a page, each domain annotation, and all of its variables, should be color-coded.
- All text in the annotations that represent variable and domain names should be capitalized. If possible, the annotations should not obstruct any text on the CRF page.
- It should include additional collection documents (e.g. questionnaires, diaries, lab print outs) if these data are NOT transcribed into the CRF.
Automating annotated CRFs
With traditional, manual annotated CRFs, you can spend a lot of time and effort:
- ensuring compliance
- identifying all annotations to be added for disparate sources
- editing the font and colour of the annotations
- adding links and bookmarks and applying changes – and often, there can be multiple rounds of changes to accommodate!
This traditional process is not only time consuming; it can result in human error and a reduction in quality of your annotations.
If you find this is the case for your current process, why not consider automating annotated CRFs to make the job a little easier?
The benefits of automating annotated CRFs:
- They’re quicker and easier to create than manual versions.
- You can achieve higher quality and more consistent annotations.
- Easily make color, position, and text consistent.
- Reuse your standardized annotations across different studies.
- Easily edit forms and annotations.
- And there’s no need to redo bookmarks – this is done automatically!
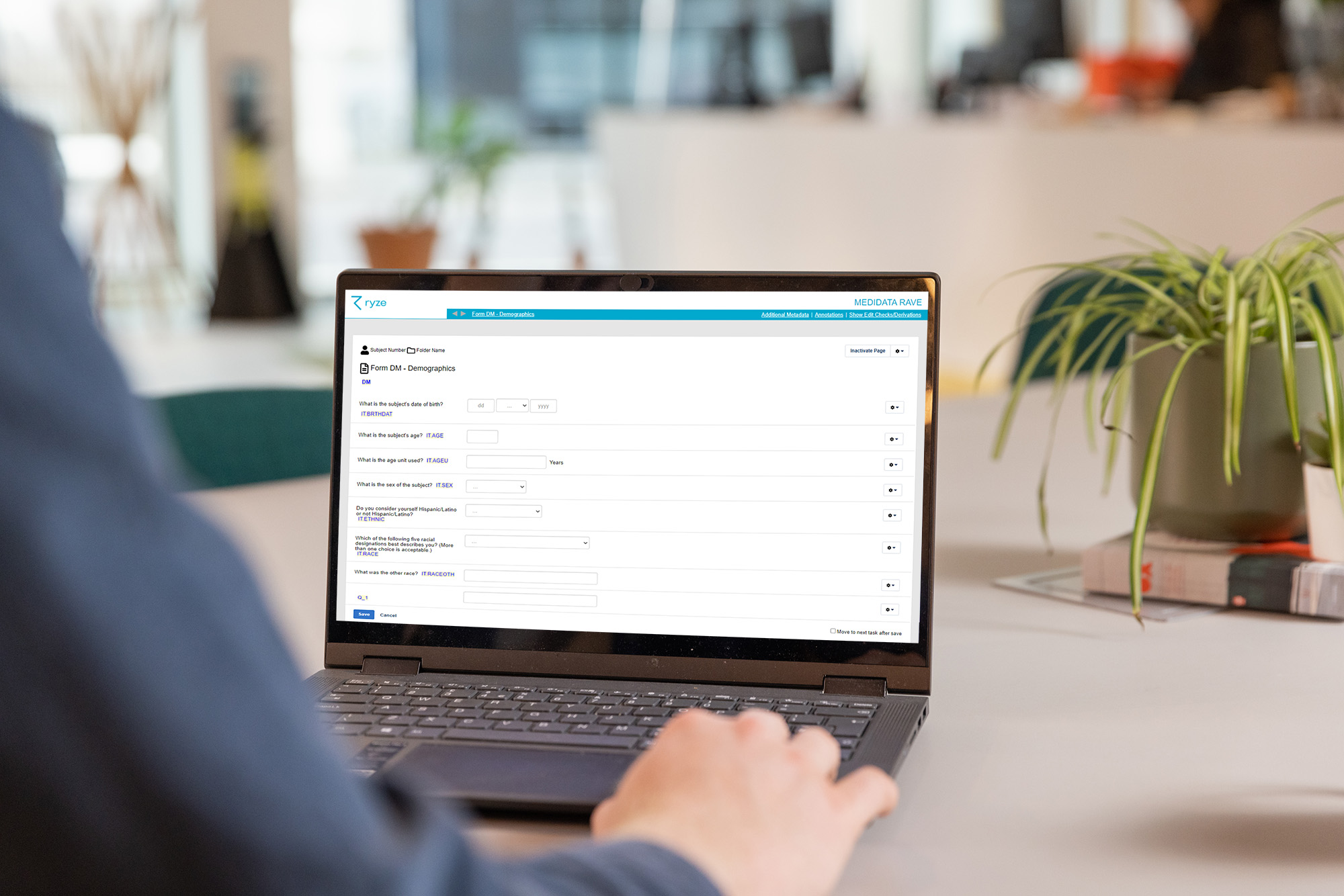
Annotated CRFs in ryze
ryze makes creating annotated CRFs easy – it’s as simple as that!
You can create your forms from scratch and add annotations. You can preview your forms and make any changes within ryze. When you’re happy, you can generate your SDTM compliant annotated CRFs at the push of a button. You know your annotations will be consistent and high quality because they’ve been standardized, and because the annotations are added to the metadata itself, they’ll also appear in your EDC, which is fantastic for approvals!
If you’re feeling overwhelmed by the challenge of creating compliant annotated CRFs, know that help is on hand.
With ryze, our all-in-one, cloud-based clinical metadata repository, you can automate annotated CRFs to save time and increase efficiencies, while ensuring that you stay compliant with industry and regulatory standards.
Our Professional Services team is here to support you. Why not get in touch now for a free, no-obligation chat about annotated CRFs.