We’re excited to announce the launch of Formedix CORE, the first free-to-use, downloadable application encompassing the CDISC Open Rules Engine (CORE).
The application officially launched in April 2023 at the CDISC Europe Interchange.
What is the CDISC Open Rules Engine?
CDISC’s mission is to raise awareness of the benefits of data standardization, to develop the highest quality data standards, and to reduce barriers to adoption of those standards.
To help achieve this, CDISC have launched CDISC CORE - an open source, freely accessible open rules engine. According to CDISC, the engine addresses the industry’s need for an ‘unambiguous set of Conformance Rules developed and tested by the CDISC Standards Development teams’.
Executable Conformance Rules are critical to the clinical study submission process, as they provide a threshold for standards conformance. Conformance Rules also make the sharing of standardized clinical data more straightforward, and enable the standardized data to be shared while the study is ongoing.
Currently, implementers of CDISC Foundational Standards are required to create their own executable rules, using CDISC’s specifications as guidance. But CDISC note that this approach can lead to inconsistencies, misinterpretations, and rules that don’t fully align with the specifications.
CDISC CORE will change things. It allows companies to easily validate against the standardized conformance rules published by CDISC. This will help stakeholders to get better visibility over the data sets and is necessary to ensuring that the data conforms with standards. Ownership of the rules and development of the CORE engine by CDISC will give sponsors the confidence that submissions will comply when submitting to the FDA.
CDISC CORE project objectives
According to CDISC, the CORE project objectives are:
- Provide clear, workable Conformance Rules for each Foundational Standard
- Ensure consistency of implementation
- Provide quick access to Rules for new Foundational Standards
- Create executable Conformance Rules vetted by CDISC Standards Development teams
- Provide a Reference Implementation of an open-source engine that executes the Rules
- Release the open-source engine under the CDISC Open-Source Alliance (COSA)
To find out more about CDISC CORE, visit the dedicated CORE page on the CDISC website.
What is Formedix CORE?
To encourage the adoption of CDISC CORE, we have developed the Formedix CORE desktop application. Formedix CORE can help you validate local study data, increase quality of data and have confidence that your submissions are CDISC-compliant.
Formedix CORE is the first desktop application to make use of CDISC CORE and its associated rule sets, which are still in development by CDISC. This means that currently, only a preview version of Formedix CORE is available. We’ve released this preview version to help you explore the technology and see how it could fit in your clinical study process.
|
Test data |
|
To make it easier for you to test out the platform, CDISC have provided test data that you can use to try out Formedix CORE and see how to validate study data in practice. The test data is included in the Formedix CORE downloadable zip file. |
How does it work?
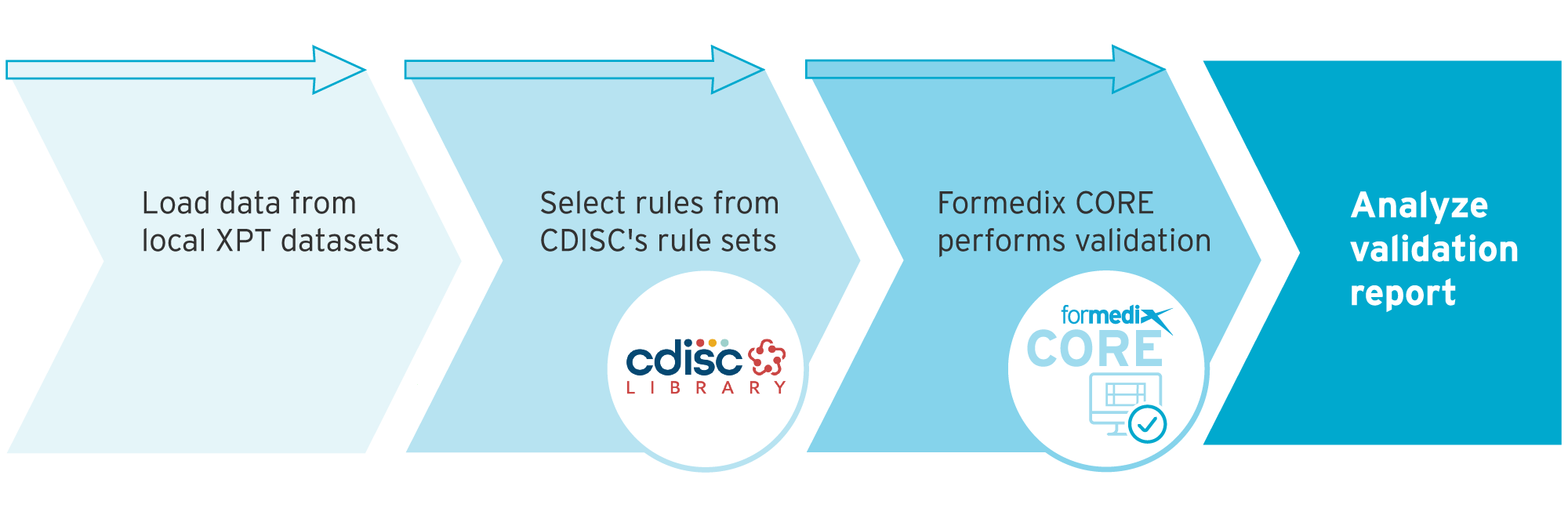
- Locate your data – Load data from your local XPT datasets.
- Select rules – Choose which rules you want to validate against from standardized conformance rules in CDISC’s Open Rules Engine e.g., SDTM-IG 3.4.
- Validate against chosen rules – Formedix CORE will validate your data against your chosen rules.
- View validation report – The report will display any conformance issues found in your datasets so these can be corrected for better data quality.
|
Your data Please note that Formedix CORE processes your data on your computer. No data leaves your computer and no data is sent to Formedix or to anyone else. On startup, Formedix CORE makes a call to a Formedix server to retrieve information about the latest version of the product. It will inform you of updates and give you the option to download them. |
Why use Formedix CORE?
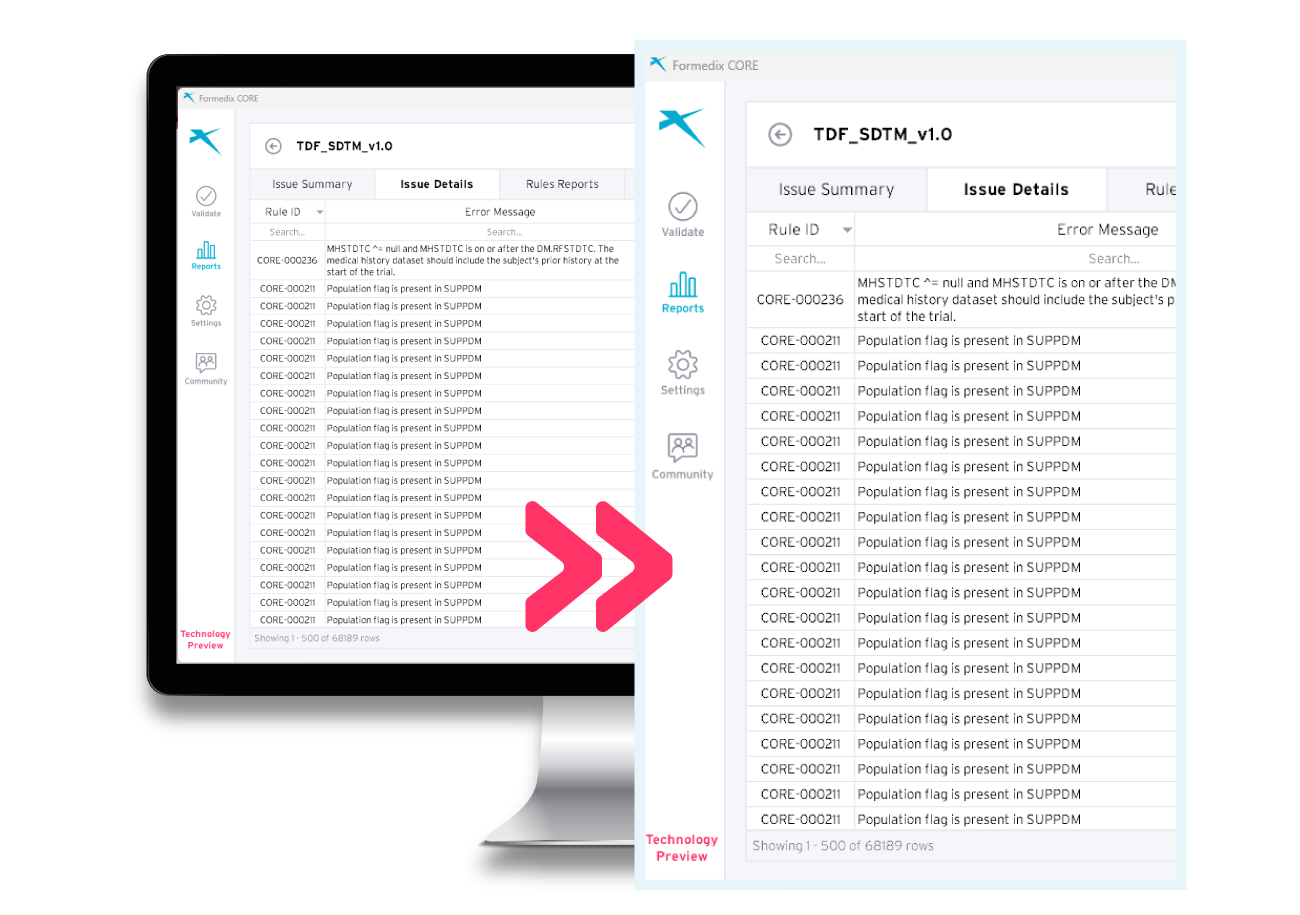
Easy-to-read validation reports
The filterable and sortable validation reports provide better visibility of your datasets. You can identify and correct issues found in the reports, resulting in better data quality. You can also save the report as an Excel file, for storing or sharing with others.
Greater quality and consistency
Using Formedix CORE, you’ll align with CDISC’s conformance rules throughout your studies. This means your data will be consistent and high quality and will be as close to submission ready as possible.
Easily update to the latest rule sets
The app contains a version of the CDISC Open Rules Engine and the latest version of CDISC’s rule sets (at time of release). You can also refresh the rule sets with the latest from the CDISC Library. And because the rule sets are built and governed by CDISC themselves, you can have confidence that you are using the best available rules.
Give feedback and help shape CORE
When you download Formedix CORE, you’ll also get access to a dedicated community forum: a place for you to collaborate with other users, expand your knowledge of CORE and share your experience of using the platform.
We encourage all Formedix CORE users to provide feedback on their CORE experience, which will directly help to shape future versions of both the desktop application and the CORE engine.
Access the forum using the ‘Community’ button on the Formedix CORE navigation panel.
The future of Formedix CORE
The CORE engine is still under development at CDISC. The following updates are expected to be made by CDISC in a future release, and will be accessible through Formedix CORE:
- Final production version of CORE engine
- Final production version of initial rulesets
- Additional rulesets (SEND, ADaM, FDA business rules)
- Validation against CDISC NCI terminology
- Validation of additional file formats, such as CSV, Dataset-XML and Dataset-JSON
- Validation against additional dictionaries, such as UNII, Med-RT, SNOMED, MedDRA and WHODrug
Current ryze users will be pleased to know that we plan to update ryze to support validating datasets against the CORE engine and rule sets. Keep an eye on our dedicated ryze page for more details!